
Ka and Kb calculations, Acid Dissociation Constant part1 Grade 12 Chemistry Power Point WITH ANSWERS | Teaching Resources
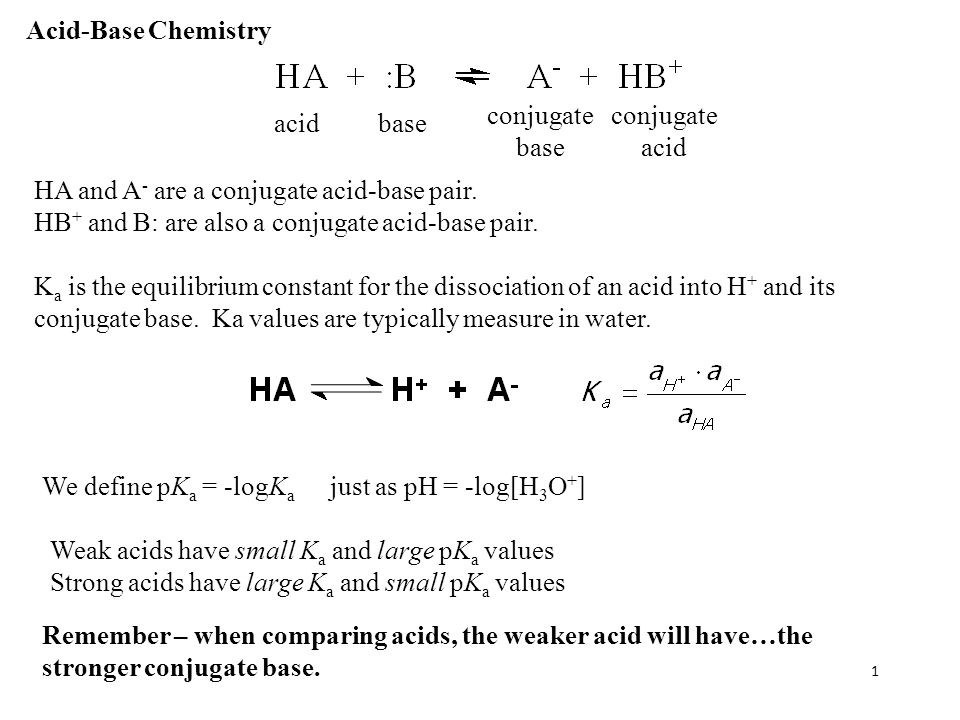
Acid-Base Chemistry K a is the equilibrium constant for the dissociation of an acid into H + and its conjugate base. Ka values are typically measure in. - ppt download
![Year 12 Chemistry: Disassosiation Constants (Ka)] Need verification on my working steps...How do we get differing final (E) concentrations?? : r/HomeworkHelp Year 12 Chemistry: Disassosiation Constants (Ka)] Need verification on my working steps...How do we get differing final (E) concentrations?? : r/HomeworkHelp](https://i.redd.it/year-12-chemistry-disassosiation-constants-ka-need-v0-mb11cqg0f3pa1.png?width=1909&format=png&auto=webp&s=e44108ebc2aeadfb66693e2efb8d8268c8cf2cb8)
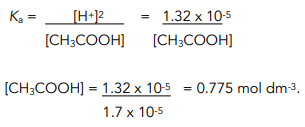




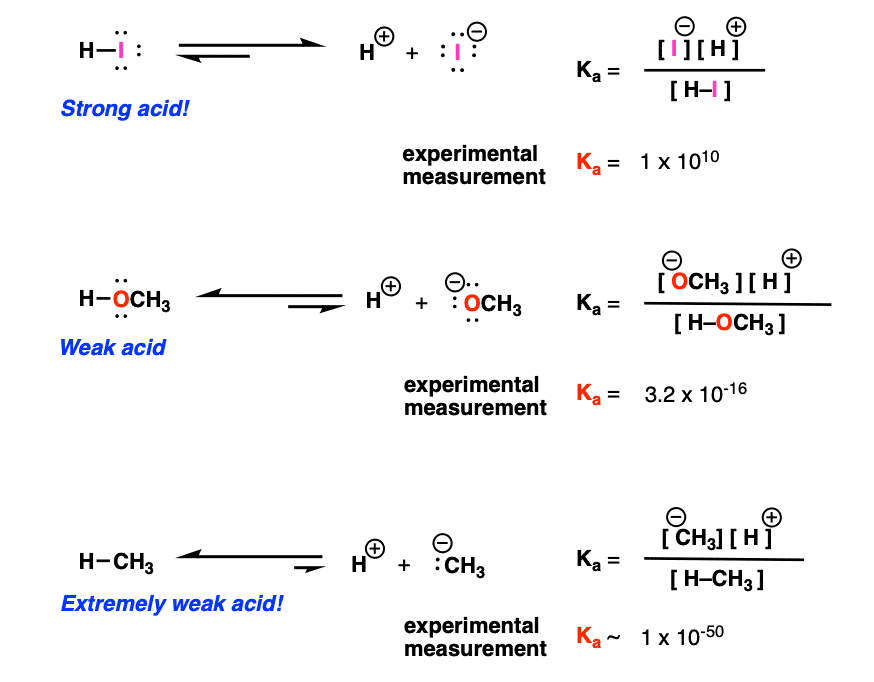
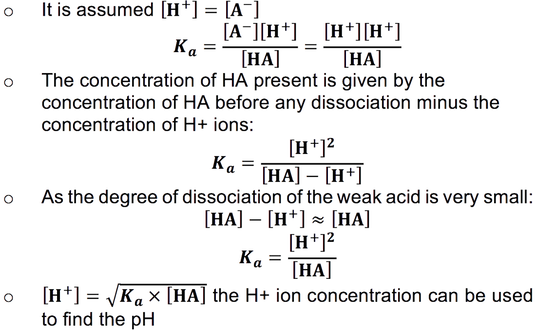

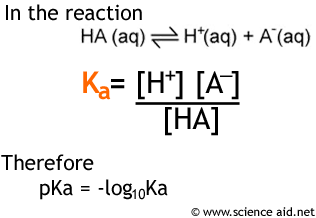
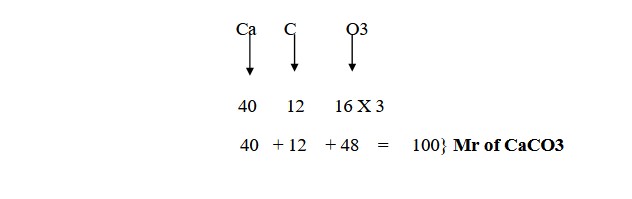
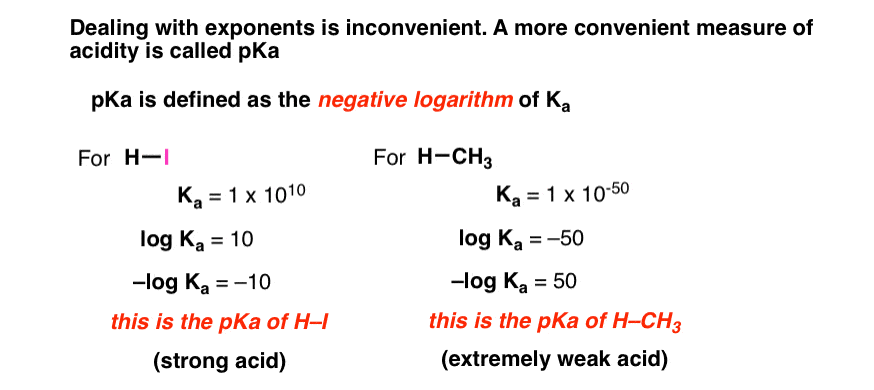








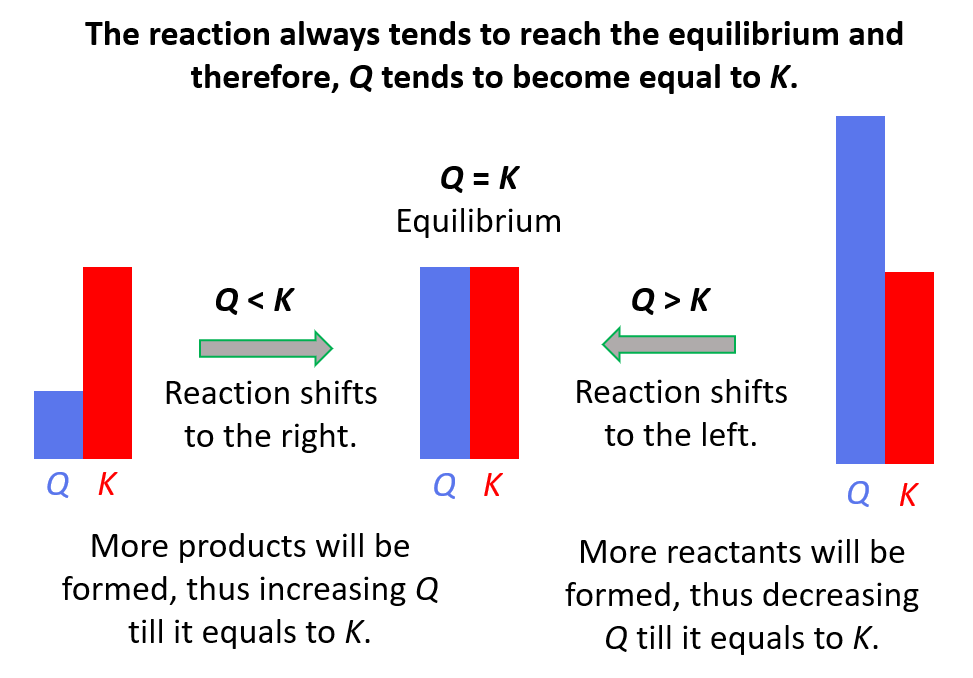
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)